Oxidizer Helps Control Highly Variable H2S Emissions


Lubrizol's LeHavre facility.
Problem
Lubrizol's Le Harve, France, facility produces a lube-oil additive in a batch-type reaction that uses elemental sulfur as a reactant and processes H2S as a byproduct.
During the facility's 8-hr reaction cycle, the H2S concentration in the reactor effluent gas ranges from zero to approximately 100%.
The facility needed a system to handle its variations in H2S concentration and to yield elemental sulfur pure enough to recycle as feedstock to the batch reactors.
The cyclic rate of H2S imposes a maximum instantaneous H2S rate of 10 mt/d, even though the average daily H2S loading is equivalent to only 3 mt/d.
Solution
Lubrizol France SARL (Paris) chose the Lo-Cat unit from USFilter/Gas Technology Products (Schaumburg, IL), which manages a large, repetitive turndown.
The Lo-Cat II process is an isothermal method for forming elemental sulfur by transferring electrons from sulfide ions to oxygen. The reaction takes place in an aqueous solution containing iron ions (USFilter chose iron over other metal-ion catalysts because it is inexpensive and non-toxic). The basic reactions are an absorption and regeneration phase.
In the Lubrizol Lo-Cat II system, sour gas from the batch reactors runs through a compressor and then though an inlet knockout pot to remove any liquid droplets. The gas subsequently is sparged into the regenerated Lo-Cat solution in the absorber sections of the auto-circulation vessel.
Rotary lobe blowers supply air that is sparged through the reduced Lo-Cat solution in the oxidizer sections of the auto-circulation vessel. The iron catalyst gets circulated throughout the vessel by an "air lift." The lift results from the differences between the aerated density of the absorbers and oxidizers and the non-aerated density of the regions between the baffles and weirs Both the H2S oxidation to sulfur and regeneration of the iron catalyst occur in the same vessel.
A pump removes a small slipstream of the resulting solution to a sulfur-settler vessel where it is concentrated into a 10 wt% sulfur slurry. Progressing-cavity pumps then deliver the slurry to a vacuum-belt filter, which produces a sulfur cake of approximately 60 wt%. The filter cake is water-washed, and the filtrate (catalyst solution) and the wash water are pumped back to the auto-circulation vessel.
The filter cake falls off the belt filter into a re-slurry tank, where water is added to produce 10 wt% sulfur slurry. Another progressing-cavity pump is used to direct the sulfur slurry from the re-slurry tank into the melter, a patented vertical shell-and-tube exchanger. The molten sulfur then flows to a separator. There, the sulfur settles to the bottom and water rises to the top. The resulting elemental sulfur then recycles back to the batch reactors.
Results
The Lo-Cat II has performed its dual processes effectively since its startup in June 1996, having reduced H2S emissions from 820,000 ppmv to less than 10 ppmv.
During each two-batch cycle, the Lo-Cat II unit produces and recycles approximately 3,000 kg of high-quality sulfur, maintaining Lubrizol's product quality.
Said US Filter VP/GM Gary Nagl, "Because the byproducts of the oxidation system are reused successfully as raw materials, this installation is truly environmentally friendly."
Lubrizol has saved money by recycling elemental sulfur, and it has improved its image by not degrading the air quality of Le Harve.
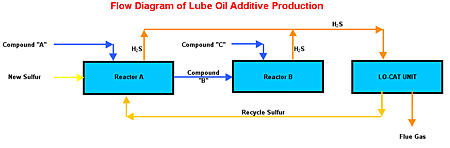
The Lubrizol batch process.
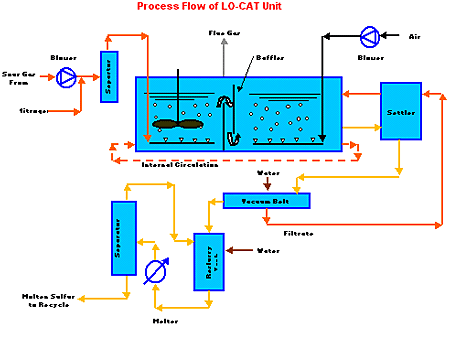
The Lo-Cat II process.
Contact: USFilter Gas Technology Products, 1501 E. Woodfield Rd., Suite 200W, Schaumburg, Illinois 61073. Tel: 847-706-6900; Fax: 847-706-6955.
The previous case study is adapted from a case study edited by Alan S. Brown, managing editor of Chemical Online, a companion site to Pollution Online within the VerticalNet e-commerce family.
