Membrane Provides VOC Recovery Alternative

Choosing a VOC control technology depends on a number of factors, including: the nature and concentration of the organic compound to be removed, the flow rate of the stream being treated, the removal and/or recovery efficiency required, specific site characteristics, and the value of the organic pollutant. Selecting the most advantageous technique from among the following treatments depends on the specifics of the process and its allowable emissions.
1. Carbon adsorption is most commonly applied to dilute mixtures of VOCs and air. Inlet concentrations greater than 5000 ppmv usually require dilution prior to adsorption. Carbon adsorption is typically most economical for dilute concentrations. Carbon adsorption is not effective for the most volatile materials and is rarely used for ketones and other highly reactive compounds because of the danger of igniting the bed.
2. Steam or hot gas is used to recover VOCs from the adsorption medium. Regeneration with steam produces a liquid waste stream's containing VOCs that may not be easily recycled or may need to be handled as a hazardous waste.
3. Condensation generally requires high inlet concentrations (above 5%) and costly low temperatures or high pressure to achieve stringent environmental requirements.
4. Membrane systems are best suited for treating streams containing more than 5000 ppmv organic vapor. System costs increase with flow rate but are relatively independent of the organic-vapor concentration.
Membrane systems are especially effective when installed upstream of the final vent, as an in-process recovery technique. In addition, the combination of membrane recovery with other air-pollution control technologies appears to have compelling advantages over other options.
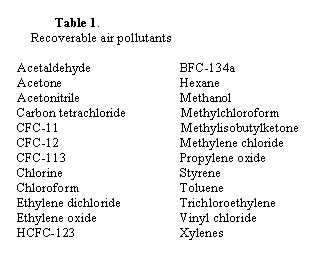
The vapor-separation process developed at MTR uses both compression-condensation and membrane separation. Such joint use creates an optimized overall process that achieves better results and higher efficiencies than either method alone. Air pollutants recoverable by the two-step process appear in Table 1.
In the vapor/compression process, a vapor/air mixture is first compressed to 45 to 200 psig. The compressed mixture then goes through a condenser and there is cooled. A portion of the organic vapor condenses and goes to a solvent-storage tank. The non-condensed portion of the vapor/air mixture passes across the surface of a composite membrane that is much more permeable to organic vapors than to air. (A pressure differential must be maintained across the membrane.)
The membrane separates the gas into two streams: a permeate stream, containing most of the remaining solvent vapor from the condenser, and a solvent-depleted stream, essentially stripped of organic vapor. The permeate stream is drawn back into the inlet of the compressor. The solvent-depleted air vents from the system.
The membrane comprises three layers: a nonwoven polymer fabric foundation; a tough, open, microporous membrane cast overlay; and, last, a very thin perm-selective coating. This last layer accounts for the membrane's separating properties.
The membranes are spiral-wound packaged. Feed gas that enters the module flows between the membrane leaves; the organic vapor preferentially permeates the membrane and spirals inward to a central permeate-collection pipe. The air flows across the membrane surface and exits as the residue. Serial- and parallel-module flow arrangements meet the capacity and separation requirements of the particular application.
About the authors: The authors are with Membrane Technology and Research, Inc., 1360 Willow Road, Menlo Park, CA 94025-1516.
Contact: Membrane Technology and Research, Inc., Marc L. Jacobs, 1360 Willow Road, Menlo Park, CA 94025-1516. Tel: 415-328-2228; Fax: 415-328-6580; E-mail: mjacobs@mtrinc.com.
Edited by Paul Hersch
